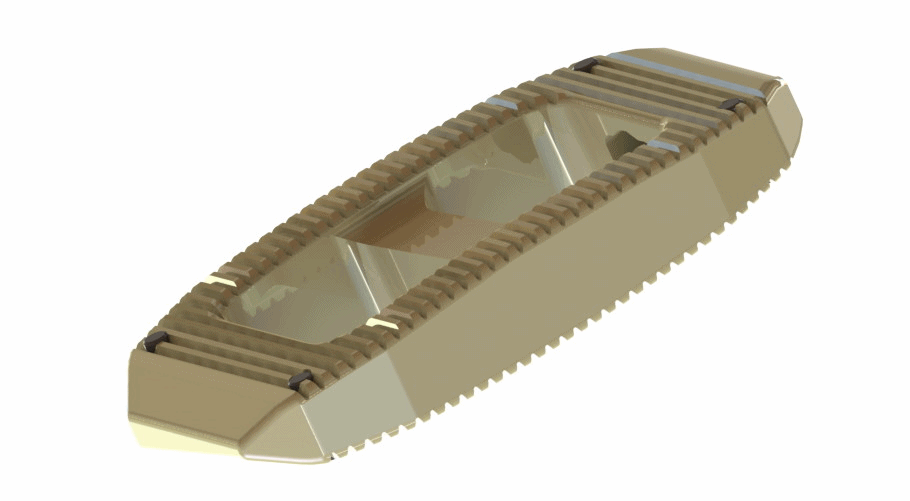
Solvay Specialty Polymers announced that TranS1 Inc, a supplier of minimally invasive spinal implants and medical devices in the US, has commercialized the VEO Direct Lateral Access and Interbody Fusion System which incorporates a lumbar fusion cage implant made of Solvay’s Zeniva polyetheretherketone (PEEK) resin. The VEO system also features a tubular retractor made of Solvay’s Radel polyphenylsulfone (PPSU) resin for radiolucency and the ability to withstand repeated steam sterilization.
TranS1’s VEO is a direct lateral fusion system for the lumbar spine. VEO’s interbody cage is made from Zeniva PEEK rod stock that TranS1 offers in various sizes, including widths of 17mm and 22mm and lengths from 40mm to 60mm. The implant has a large center channel to allow bone growth through the device, fusing the adjacent bony surfaces of the vertebrae.
Through a combination of direct psoas visualization and clear lateral fluoroscopic views, the VEO Direct Lateral System offers complete visualization of the operative site. This approach was designed to help minimize iatrogenic trauma to the psoas muscle and the nerve plexus to help reduce the risk of post-operative complications, according to Solvay.
The VEO Direct Lateral System offers interbody implants in both parallel and lordotic angles to match each patient’s anatomy. The interbody implants made using Zeniva PEEK contain five tantalum markers for precise fluoroscopic visualization. The large center channel is readily visualized and can be easily evaluated for progression of fusion.
Zeniva PEEK, part of Solvay’s line of Solviva Biomaterials, has a modulus close to that of bone plus strong toughness and fatigue resistance. It is certified to meet the full requirements of the ASTM F2026 standard for PEEK used in implantable surgical devices.
As said, it is a comparable or better-performing alternative to metals such as titanium for these intervertebral implantable devices. Based on biocompatibility testing, Zeniva PEEK demonstrates no evidence of cytotoxicity, sensitization, irritation, or acute systemic toxicity. It also boasts high strength and stiffness and has radiolucent properties which enable x-ray procedures without interference.
“This is a perfect example of the value Solvay brings with its breadth of products, expanding the options for designers in terms of design flexibility and performance optimization,” said Shawn Shorrock, Global Healthcare Market Manager for Solvay Specialty Polymers. “In addition, the ongoing acceptance of Zeniva PEEK has validated our approach to the spinal market and we’re encouraged by the momentum we’ve generated.”
Meanwhile, the Radel PPSU used for the tubular retractor is designed to prevent soft tissue intrusion. Solvay notes that it provides superior strength, high thermal performance, chemical resistance, and the ability to withstand repeated steam sterilization. The retractor is made from 50mm diameter Radel PPSU rod stock in lengths of 100mm, 120mm, and 140mm.
The manufacturing site for Zeniva PEEK and other Solviva Biomaterials in Alpharetta, Georgia, the US, is ISO 13485 registered and the relevant aspects of current Good Manufacturing Practices are also applied. In addition, the company provides product traceability and all materials are tested in an ISO 17025 accredited lab.
Source: http://www.adsalecprj.com/Publicity/MarketNews/lang-eng/article-67003543/Article.aspx